LECANEMAB
- Home
- LECANEMAB
What is lecanemab?
Lecanumab is a monoclonal antibody developed by Biogen through partnership with Eisai pharmacuticals that removes a protein called beta-amyloid from the brain. It is infused through a vein for about 1 hour every two weeks for a total of 18 months.
Patients with Alzheimer’s disease have a build-up of the proteins, beta-amyloid and tau in their brain. The accumulation of these proteins is thought to result in the degeneration of neurons and the associated cognitive decline. With the help of your immune system, lecanumab selectively targets and removes the beta-amyloid from the brain.
Lecanemab Phase 3 Trial: Clarity
Lecanemab slows cognitive decline in patients with Alzheimer’s disease
Commentary
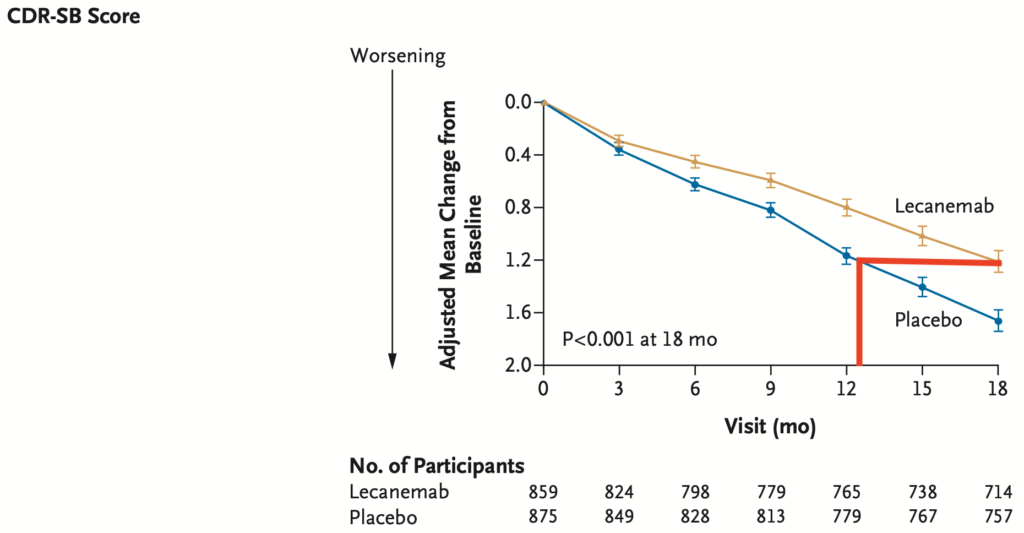
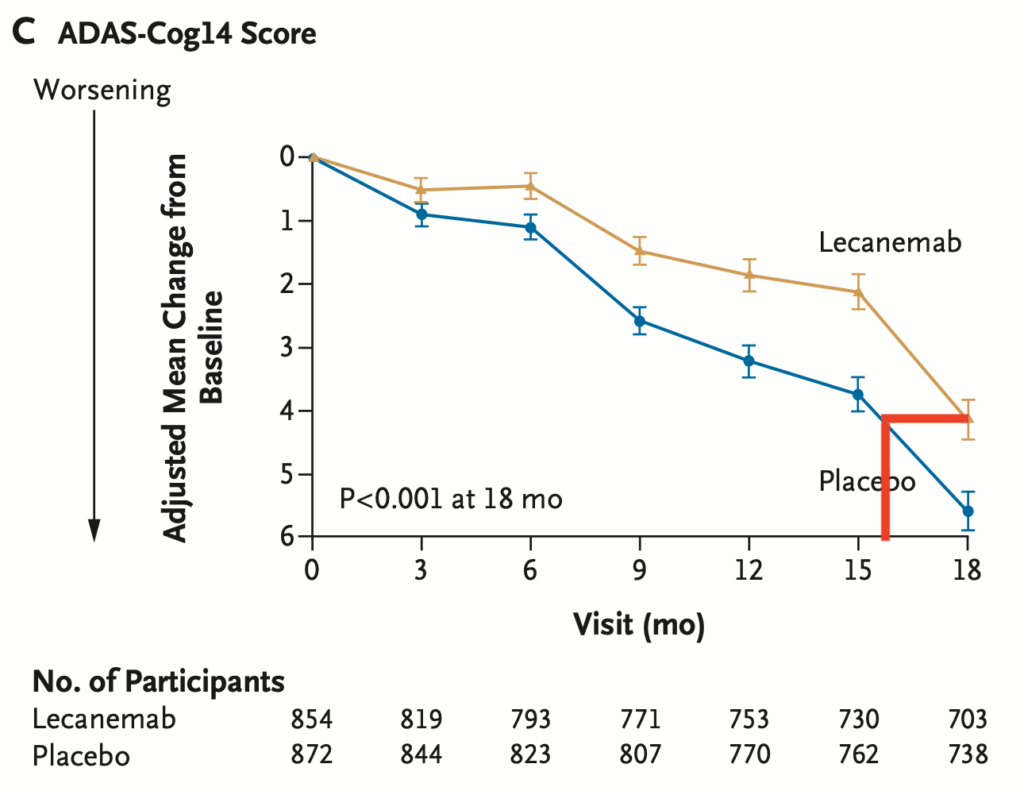
Patients do not get better on lecanemab and lecanemab does not stop the decline.
Lecanemab only slows cognitive decline (as detected by the ADAS-Cog14) and activities of daily living (as detected by the CDR-SB) over the course of 18 months by about 30%. Whether this slower rate of decline is permanent or worsens after stopping lecenemab is not known.
Only patients with Mild Cognitive Impairment or Early Dementia from Alzheimer’s Disease were enrolled in this study.
Another way to think about this is that patients on lecanemab only declined the equivalent of 16 months cognitively (ADAS-Cog14) or 13 months functionally (CDR-SB) over the 18 months of treatment.
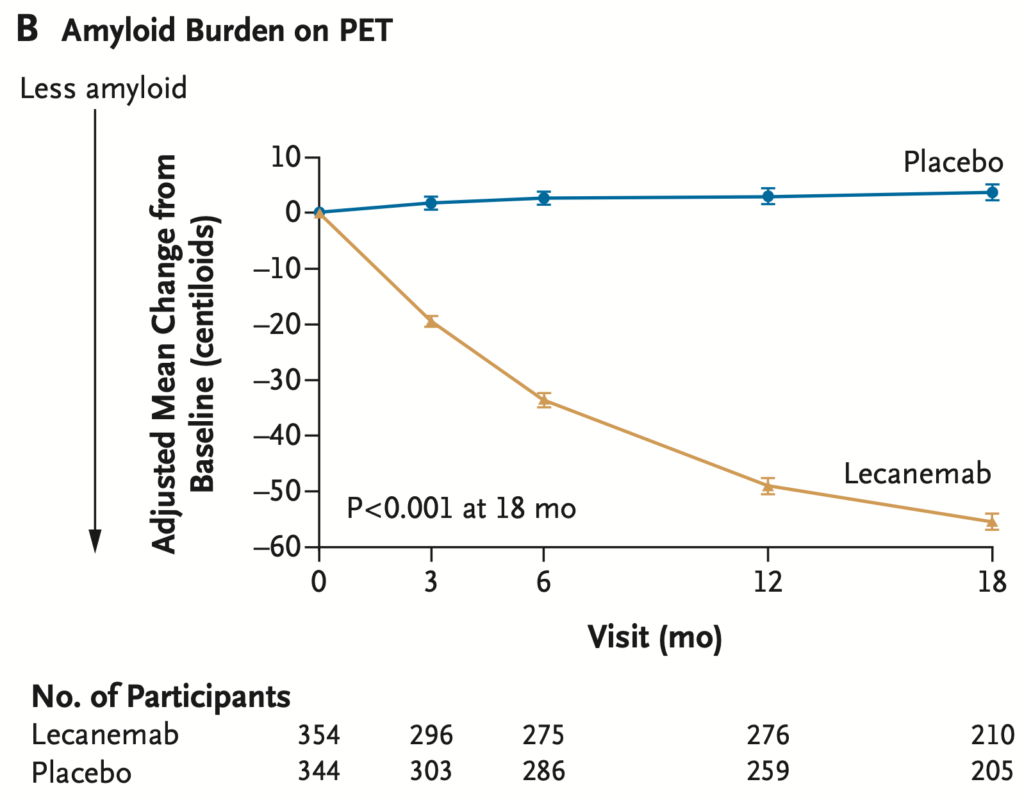
Lecanemab removes amyloid from the brain
Commentary
There is no evidence that those patients with the most amyloid removed had the most benefit clinically. We do not know the significance of the amyloid removal.
Which patients benefit the most from lecanemab?
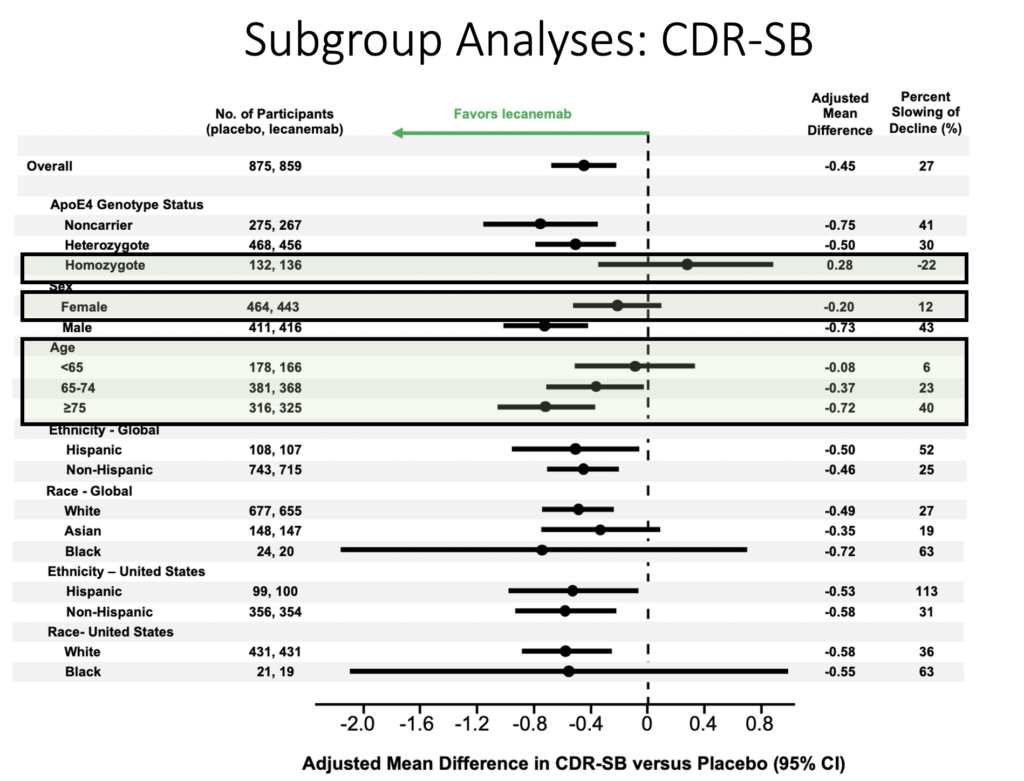
Commentary
Male patients, ApoE4 noncarriers, and patients older than 75 responded better to lecanemab.
Conversely ApoE4 carriers and patients younger than 65 did not respond as well to lecanemab.
African Americans/Blacks had a highly variable response to lecanemab.
Adverse Events
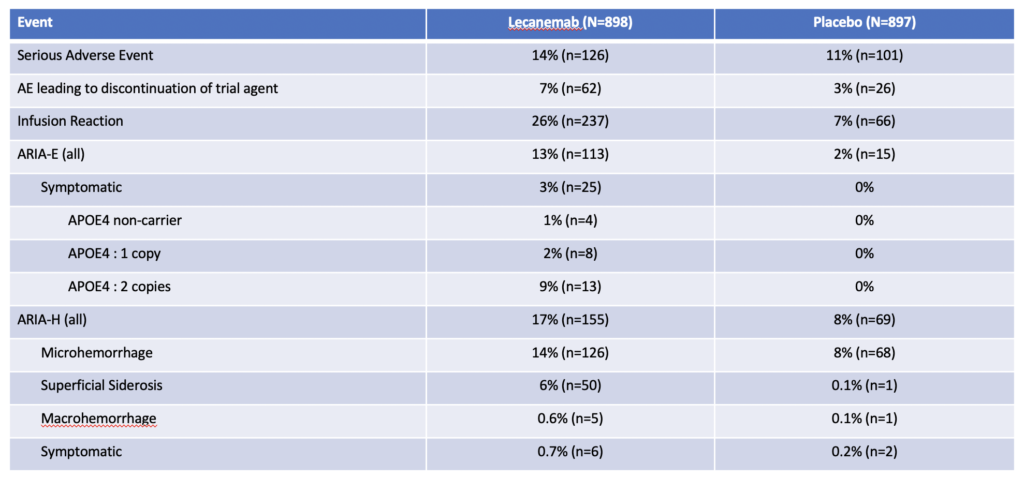
Commentary
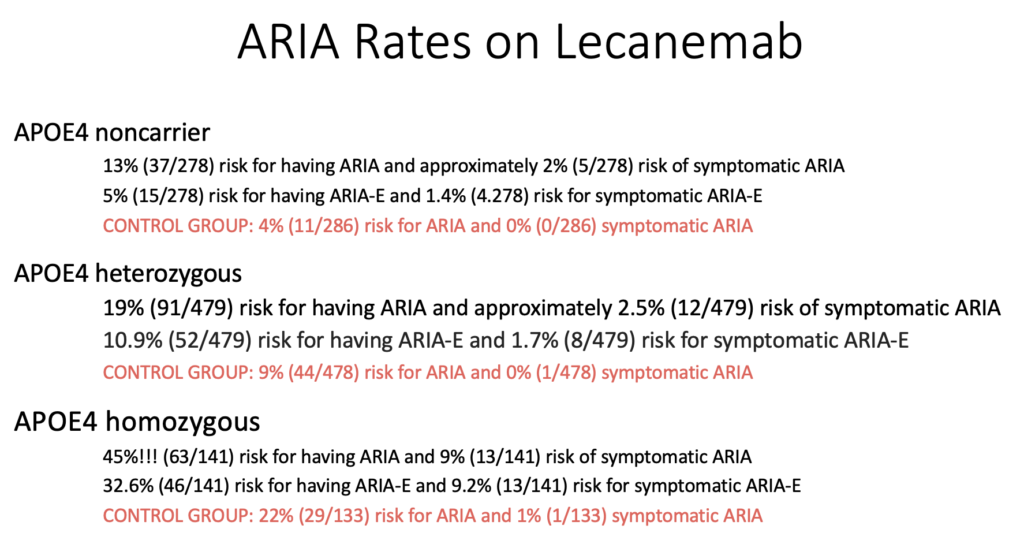
The most concerning side effect was ARIA – Amyloid Related Imaging Abnormalities. This is either swelling (ARIA-E) or bleeding (ARIA-H) in the brain.
13% of patients receiving lecanemab will develop ARIA-E and about 1/5 of these patients will have symptomatic ARIA-E requiring stopping lecenemab. Symptoms include headaches, visual problems, and confusion.
17% of patients will develop ARIA-H and only 4/100 will have symptomatic ARIA-H requiring stopping lecanemab. Most common symptom was dizziness.
Patients with two copies of the ApoE4 allele have the highest risk of developing ARIA.
About one quarter of patients will have an infusion reaction. Most will be a single reaction during one of the first few infusions. The vast majority can be treated with medications and patients were able to continue their infusions.
There have been 2 fatalities from brain bleeding. One patient was taking an oral anticoagulant and the second patient had a stroke and received TPA, a clot busting agent.
And there has been 1 fatality from brain swelling and edema in patients who received lecanumab.
FAQs
What is lecanemab?
Lecanumab is a monoclonal antibody developed by Biogen through partnership with Eisai pharmacuticals that removes a protein called beta-amyloid from the brain. It is infused through a vein every two weeks.
How does lecanemab work?
Patients with Alzheimer’s disease have a build-up of the proteins, beta-amyloid and tau in their brain. The accumulation of these proteins is thought to result in the degeneration of neurons and the associated cognitive decline. With the help of your immune system, lecanumab selectively targets and removes the beta-amyloid from the brain.
How much does lecanemab cost?
Lecanumab will cost $26,500 annually of which 80% will be covered Medicare. Additional costs such as, but not limited to, amyloid PET and MRI scans and related blood tests are not included in the cost.
Who is eligible to receive lecanemab?
Lecanumab was shown to be effective in the treatment of mild cognitive impairment or mild dementia stage of disease caused by Alzheimer’s. There is no evidence that it would be effective in the treatment of other types of diseases or in later stages of Alzheimer’s disease. Patients with mild cognitive impairment or mild dementia from Alzheimer’s disease with confirmed amyloid pathology, through either a lumbar puncture or amyloid beta positron emission tomography (PET) scan of the brain, will be eligible.
What are some potential risks of lecanemab?
Patients who receive lecanumab are at risk for developing a kind of brain inflammation called amyloid-related imaging abnormalities (ARIA), which involves brain bleeding, brain swelling, or a combination of the two. A few of these will need to discontinue their treatment due to concerns related to ARIA. Patients with two copies of the Apolipoprotein E4 allele are at very high risk for bleed and edema. Before and after lecanumab is started, patients must receive special monitoring (including brain MRI scans) to screen for ARIA.
There have been 2 fatalities from brain bleeds and 1 fatality from brain swelling and edema in patients who received lecanumab.
What if I am enrolled in a clinical trial?
We would encourage you to continue participation in ongoing clinical trials. If you have any questions or concerns about how starting lecanemab may impact your continued participation in the clinical trial, please discuss them with the principal investigator of your clinical trial or your physician.
What is the difference between lecanemab and donanemab?
Both lecanemab and donanemab are monoclonal antibodies directed against amyloid and work similarly.
Lecanemab is infused every 2 weeks while donanemab is infused monthly. After 18 months, lecanemab will switch to monthly subcutaneous injections (done at home). Donanemab infusions are continued until the patients’ amyloid-PET scan becomes negative.
Benefits are similar with both slowing decline by about 30%
Major risk for both medications being infusion related reactions and ARIA. Donanemab has about 1/2 risk for infusion reactions and double the risk for ARIA.
Next steps at BIDMC
The CNU is offering lecanemab to eligible patients through our DIAD clinic. To request an appointment, please signup through our on-line enrollment list at